In sewage treatment, phosphorus content is one of several key control indicators in sewage treatment. Phosphorus includes total phosphorus, organic phosphorus, and inorganic phosphorus. Inorganic phosphorus salts such as phosphates, polyphosphates, and polyphosphoric acids in sewage, and organic phosphorus such as phospholipids. Phosphorus is an essential element for the biological treatment of sewage, and one of the elements that cause water eutrophication. All methods of phosphorus removal from wastewater contain two necessary processes, first to convert dissolved phosphorus (phosphate) substances into an insoluble suspended (granular) state, and then to remove phosphorus from wastewater by solid-liquid separation. This process is generally accomplished through the use of phosphorus removal agents. Phosphorus remover is the addition of chemicals to sewage to make phosphate ions in the water form insoluble salts, form flocs, and then separate from the water, thereby removing phosphorus contained in the water.
Polyaluminum chloride is a commonly used phosphorus remover. In the specific reaction process, there are two main reaction processes, the first is that the trivalent aluminum ion reacts with phosphate to precipitate, and the precipitated compound is AlPO4.
Al3+ + PO4- → AlPO4
Secondly, the trivalent aluminum ion can undergo a hydrolysis reaction. In this process, there will be positive charges and the existence of mononuclear hydroxyl complexes and polynuclear hydroxyl complexes. After a series of actions such as van der Waals force and net capture, It can achieve a relatively ideal precipitation effect, thus meeting the requirements of chemical phosphorus removal.
In the process of using polyaluminum chloride for chemical phosphorus removal, how to improve the phosphorus removal ability?
(1) It is necessary to focus on pH control, so as to achieve the ideal phosphorus removal effect.
PH will affect the production of AlPO4 precipitation, and also affect the flocculation effect of polyaluminum chloride, so the effect of controlling PH is great.
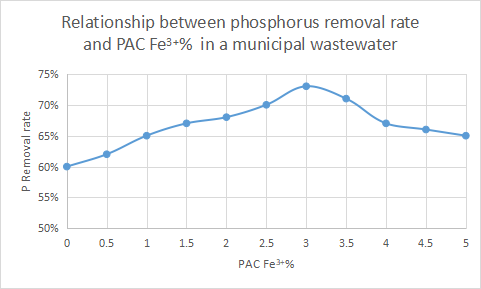
(2) Polyaluminum chloride containing certain iron can be selected, or polyaluminum ferric chloride can be selected.
The precipitate formed by the reaction of iron salt and phosphate is more stable than aluminum salt, and has the advantage of fast sedimentation, so containing a certain amount of iron salt can improve the phosphorus removal ability. Polyaluminum ferric chloride can play the advantages of iron salt for phosphorus removal, and it can also play the advantages of polyaluminum chloride, which is an excellent new type of phosphorus removal agent.

Leave A Comment